The genotype of an embryo controls phenotype via the developmental process. Genes regulate development BUT, genes do not exist in isolation, they exist in the environment of the cell. An important question in developmental biology is the relative contribution of the egg cytoplasm (regulated by the maternal genome) versus the zygotic genome. Another related question of importance is how cells differentiate during development to stably express a specialized subset of genes relevant to their specialized functions, e.g., neurons, muscle cells, liver cells, etc. Is DNA irreversibly lost or altered during differentiation in response to cytoplasmic signals?
MAJOR Question of Development: Do all cells have the same DNA? This is termed the theory of Genome Equivalence.
Ideal experimental proof of Genomic Equivalence would be to transplant the nucleus from an adult differentiated cell into an enucleated egg. Will nuclei from all differentiated somatic cells support normal development? If true, then the DNA in all fully differentiated somatic cells is equivalent and the process of differentiation proceeds by fully reversible changes to the DNA.
John Gurdon and Shinya Yamanaka win 2012 Nobel Prize in Physiology or Medicine
Amphibian Cloning (John Gurdon, 1975)
The techniques for nuclear transplantation were developed by John Gurdon. He chose an amphibian, the leopard frog ((Rana pipiens ) as a model system because of the large size of the egg (the frog had long been a model system to study early development because of size, availability and ease of rearing animals in the lab). Using small glass micropipettes he hand fabricated with a microforge he developed techniques for:
 1. Enucleating eggs
1. Enucleating eggs
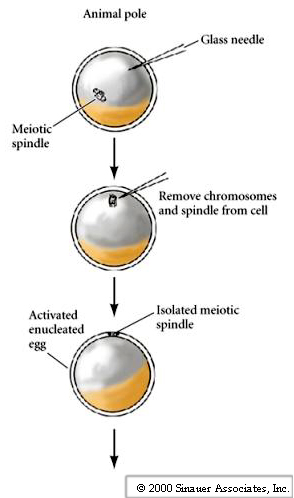
2. Transferring in a new nucleus.
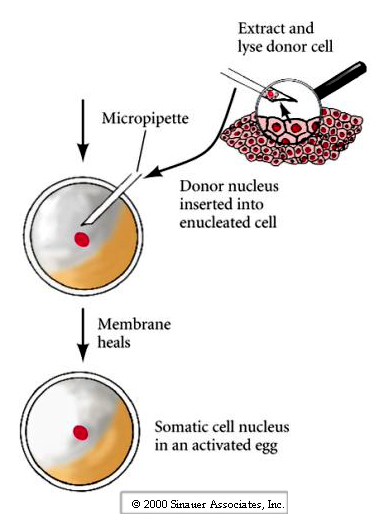
3. Activating the developmental program in the egg.
As you can see from the figure the early blastula nuclei are the best at supporting normal development to the tadpole stage (80% of the time).
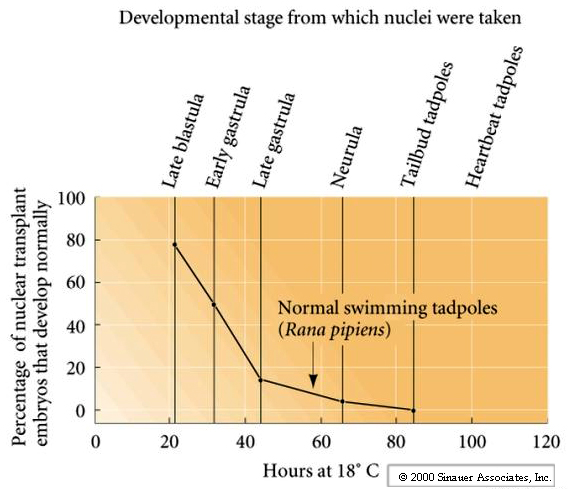
Late embryonic and larval nuclei support development to the tadpole stage in less than 1% of the transplantations. These nuclear transplantation experiments supported the hypothesis of Genome Equivalent, but also suggested that the changes occurring in the genome during development and differentiation were difficult to undo. No fertile adults were obtained.
The question still remained: Can fully differentiated somatic cells support normal development when transplanted into an enucleated egg? Further nuclear transplantations studies were done with another more primitive frog (Xenopus laevis) that develops at a much faster rate than the leopard frog. In addition, the idea that the egg cytoplasm reprograms the nuclei was further tested by serially transplanting nuclei from somatic cells to eggs, allowing them to divide to the blastula stage, and then transplanting them back to an enucleated egg. This significantly improved the success rate and led to the development of adult frogs from transplanted intestinal nuclei. Frogs were still sterile. Neuronal nuclei still would not support normal development.
Nuclei from intestinal epithelial cells transplanted into enucleated Xenopus eggs gave rise to feeding tadpoles in about 1% of the transplantations. BUT, when serial nuclear transplantations were performed the success rate increased to about 10%.
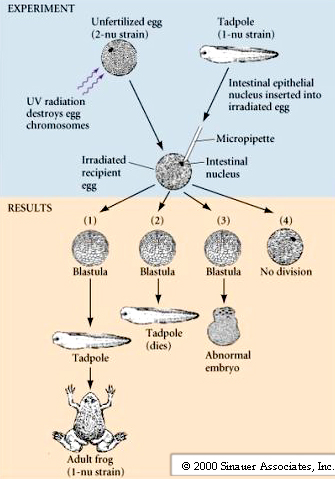
Conclusion – Stronger support for theory of Genomic equivalence. Cellular differentiation occurs through reversible changes to DNA. Exposure to egg Cytoplasm is able to cause nuclear reprogramming of nucleus from differentiated somatic cell.
The partial success of cloning frogs led to many attempts at cloning mammals, all unsuccessful until Dolly. In 1997 the scientific world was pleasantly surprised by the first mammalian clone produced by nuclear transfer of an adult somatic cell nucleus.
Ian Wilmot and his colleagues produced Dolly (see http://www.synapses.co.uk/science/clone.html for humorous account)
Sheep were cloned from somatic cell nuclei of adult female sheep. Cells from mammary glands were dissociated and grown in culture. The tissue culture media was adjusted to starve cells and maintain them in the G zero stage of the cell cycle. This was probably the key difference that led to success for this group. Most scientists that had tried cloning mammalian cells assumed that using a nucleus from a rapidly dividing cell would work best since nuclei in early embryos normally undergo rapid divisions. Fusion of enucleated oocyte with mammary cell was produced with an electrical pulse that also activated development in the oocyte.
434 attempts for 1 success! So it is still a very inefficient process. Since the successful cloning (nuclear transfer) that produced Dolly many other mammals have been successfully produced by cloning (cows, pigs, cats, mice, humans NOT YET). However, the success rate is still very low ~ 1 – 5%.
Because of the low success rate there are still questions concerning the nature of the somatic cells. It may be that endogenous stem cells are the source of nuclei for successful clones. Still no success with neurons.
Some helpful definitions:
Transgenic animal: animal that expresses a transgene; a foreign gene inserted into the genome by using recombinant DNA and in vitro cloning techniques.
Reproductive Cloning: Using nuclear transfer and cloning techniques to generate a genetically identical animals.
Therapeutic Cloning: Using recombinant DNA and cloning techniques to generate genetically engineered or defined totipotent or pluripotent stems cells for treatment of diseases, or production of tissues for transplantation.
Why Clone Mammals?
It can be hard to produce large amounts of human proteins for therapeutic purposes. One possibility is to insert a transgene (human) for the desired protein into cow or sheep under the regulatory control of an engineered regulatory domain so it will be secreted in milk (eg. Lactalbumin). In this way large quantities of the desired human protein will be secreted into the milk and easily purified.
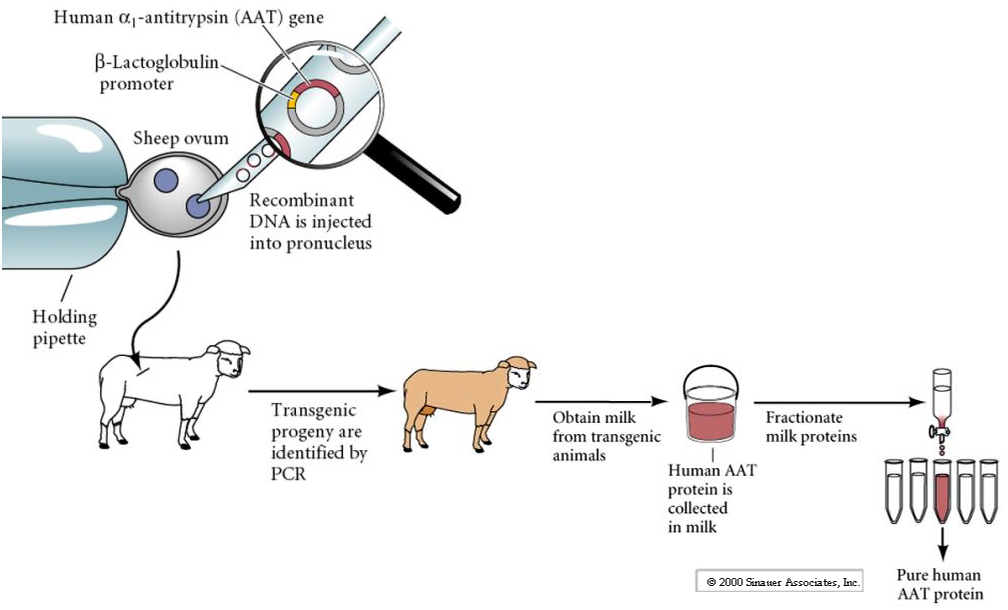
However, transgenic mammals made via DNA injection into pronucleus can be difficult to make and the transgene is often unstable. Expression levels are variable and often change in subsequent generations.
Cloning allows more precise genetic engineering of cells – and rapid expansion of "perfect" animals. In agriculture that means clones of the "best" milk producing cow or the derby winning horse.
In Humans it means true cures of genetic diseases, eg. Cystic Fibrosis. However there is also the potential for human engineering of any genetically characterized human trait
Therapeutic Cloning could be used to cure some human diseases. STEM Cells are potential therapies for many diseases such as diabetes, Parkinson’s disease, heart disease and many more. There is the potential to use genetic material from a patient’s own cells to generate perfect replacements for damaged tissue.
Why is cloning so difficult? Remember that the "sucess" rate is very low (less than 1%).
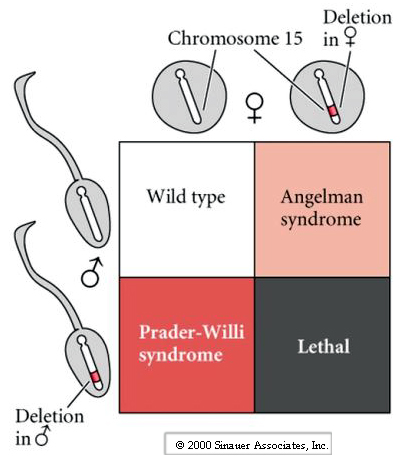
Most common phenotypes observed in Nuclear Transfer cloned animals are fetal growth abnormalities, including increased placental and birth weight. Remember that DNA is changed during differentiation and furthermore we know that during gametogenesis specialized changes occur in the male and female gametes. Genetic studies and naturally occurring human genetic diseases have shown that in some cases the disease phenotype varies dramatically depending on whether the mutated gene is from the male or female gamete. One example described in the book is a deletion on chromosome 15. If a child receives the defective chromosome from the father it gets Prader-Willi syndrome (mild retardation, obesity, small gonads, and small stature), while if the child receives the defective chromosome from the mother it gets Angelman syndrome (severe retardation, seizures, lack of speech).
It has been known for many years that the sperm and egg nucleus are not equivalent in their ability to support development.
If a sperm fertilizes an egg missing the female pronucleus the result is called an hydatiform mole and consists of placental but no embryonic tissue. An egg will sometimes spontaneously activate without being fertilized by a sperm. The parthenogenic activation leads to the development of a small disorganized embryo and a small placental. Both of course are early embryonic lethal.
It turns out that during differentiation of the gametes the DNA is specifically altered in a sex (sperm and egg) specific way that leads to differential gene expression in the genes of the two gametes. The term for this is imprinting and is the result of differential methylation of the regulator regions of genes. Thus the patterns of methylation during gametogenesis are distinct in the in the sperm and egg nucleus.
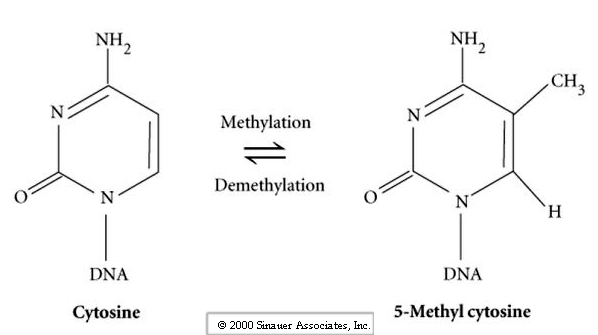
Why are the male and female pronuclei non-equivalent? Answer may lie in the genetic conflict between the male and female.